
Extracellular RNAs as communicators in cardiovascular disease: a narrative review
Introduction
Cardiovascular disease (CVD) is the leading cause of global mortality with ~18 million deaths each year (1). Due to the socio-economic burden resulting from CVD, many approaches have been taken to uncover the molecular mechanisms underlying different etiologies of CVD. The common features within various etiologies [e.g., heart failure (HF) after myocardial infarction (MI)] is cell death, which is regulated by complex networks of signaling pathways (2). Furthermore, increasing evidence suggest the complex cell-cell communication among different cell types during the remodeling of the heart after injury (3). For example, MI leads to loss of heart muscle (cardiomyocytes) that is replaced by non-contracting scar tissue (4). Although healing after MI is necessary for scar formation, it is often accompanied by adverse remodeling of the remaining viable myocardium and progression of pump failure. The immune system, in which macrophages play a key role, is a double-edged sword in the remodeling process (5). Macrophages are necessary for repair in the acute phase as their systemic depletion results in impaired scar formation and left ventricle (LV) rupture (6). However, their accumulation in non-infarcted regions of LV in the chronic phase contributes to progressive myocyte attrition, collagen deposition by myofibroblasts (activated fibroblasts), and loss of the pump function. Thus, understanding the cell-cell communication during the remodeling of the heart is important in uncovering the disease mechanisms of CVD.
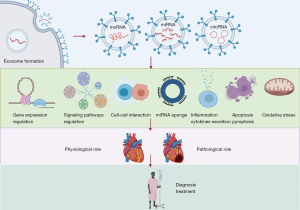
Extracellular RNAs (exRNAs) are produced by a donor cell and are released into the extracellular environment (e.g., body fluid, circulation) (7). ExRNAs could be received by recipient cells, where exRNAs may function as cell-cell communication materials. They can be found to be contained in the lipid particles, such as extracellular vehicles (EVs), including exosomes. ExRNAs include protein-coding mRNAs and non-protein-coding RNAs, including transfer RNAs (tRNAs), small interfering RNAs, microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). Due to the availability of commercial products to isolate EVs and RNA sequencing (RNA-seq) to characterize exRNAs, this field of study has exploded in recent years, including exRNAs in CVD (8). In this narrative review, we will summarize the recent developments in this field by particularly focusing on their functional and mechanistic roles (Figure 1). We present the following article in accordance with the Narrative Review reporting checklist (available at https://exrna.amegroups.com/article/view/10.21037/exrna-22-3/rc).
Methods
A literature review was performed using PubMed to search all scientific articles published through to April 26, 2022. The search terms used included: “extracellular”, “heart”, “cardiovascular disease”, “RNA”, “miRNA”, “circRNA”, “lncRNA”. We use a table (Table 1) to present detailed search strategy. Focus was placed on original articles in English about exRNAs in cardiovascular system and disease; it excluded articles that have no information about cardiac exRNAs.
Table 1
| Items | Specification |
|---|---|
| Date of search | April 26, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | Extracellular, heart, cardiovascular disease, RNA, miRNA, circRNA, lncRNA |
| Timeframe | January 3, 2014–April 26, 2022 |
| Inclusion and exclusion criteria | Focus was placed on original articles in English about exRNAs in cardiovascular system and disease; it excluded articles that have no information about cardiac exRNAs |
circRNA, circular RNA; lncRNA, long non-coding RNA; exRNAs, extracellular RNAs.
Extracellular miRNAs in CVD
MiRNAs are short RNAs with ~22 nucleotides (nt) in their mature form that regulate mRNA decay and translation by binding to the 3'-untranslated region (UTR) of mRNAs (9). Typically, one miRNA has several hundreds of mRNAs as its targets and is conserved across species. Furthermore, miRNAs are rather stable in the circulation, which are investigated as diagnostic biomarkers for various diseases, including CVD (10-13). Because of their stabilities, they are often found as exRNAs (14,15) (Table 2). For example, exosomes derived from angiotensin II-induced hypertrophied neonatal rat cardiomyocytes induced the secretion of inflammatory cytokines, IL-6 and IL-8, when co-cultured with the murine macrophage cell line RAW264.7 (16). RNA-seq analysis revealed that miR-155 was enriched in exosomes derived from hypertrophied cardiomyocytes compared to non-induced cardiomyocytes. In vitro, it was shown that exosomal miR-155 regulates the MAPK signaling pathway via the phosphorylation of p38, ERK1/2, and JNK, to activate macrophages. Not only cardiomyocytes secrete miRNAs as exRNAs, but also other cell types in the heart, especially those in diseased hearts, regulate changes in cellular phenotypes triggered by miRNAs as exRNAs. For example, the same miRNA, miR-155, was shown to be released in exosomes from macrophages in a uremic mouse model by removing parts of kidneys to study uremic cardiomyopathy (17). Using miR-155 knockout mice compared to wildtype C57BL/6 mice, it was demonstrated that exosomal miR-155 was released into the cytosol of cardiomyocytes, which targets the forkhead transcription factor, FoxO3a, to promote cardiomyocyte pyroptosis (pro-inflammatory programmed cell death) and uremic cardiomyopathy changes, such as cardiac hypertrophy and fibrosis. It is rather paradoxical that the same miRNA is shown to be released as exRNAs from both cell types that these two studies reported to influence the phenotypic changes in each other, which highlights the confusing findings in this field of study.
Table 2
| RNA | Sources | Phenotypes/mechanisms | Reference |
|---|---|---|---|
| miR-155 | Angiotensin II-induced hypertrophied neonatal rat cardiomyocytes | Regulates the MAPK signaling pathway via the phosphorylation of p38, ERK1/2, and JNK, to activate macrophages | (16) |
| miR-155 | Macrophages in a uremic mouse model by removing parts of kidneys to study uremic cardiomyopathy | Targets the forkhead transcription factor, FoxO3a, to promote cardiomyocyte pyroptosis and uremic cardiomyopathy changes | (17) |
| miR-25-3p | Mesenchymal cells isolated from murine bone marrow | Targets the pro-apoptotic Fasl and Pten mRNAs to regulate their protein levels as well as Ezh2, in turn derepress the cardioprotective gene eNOS and anti-inflammatory gene Socs3 | (18) |
| PVT1 | Angiotensin II-induced cultured human cardiac cardiomyocytes | Functions as miRNA sponge to sequester miR-145-5p in macrophages to promote its polarization to pro-inflammatory M1 macrophages | (19) |
| MALAT1 | Endothelial cells | Interacts with the transcription factor NRF2, thereby inhibits the accumulation of reactive oxygen species and maturation of dendritic cells | (20) |
| MALAT1 | Endothelial cells | Affects the formation of neutrophil extracellular traps in a mouse model of atherosclerosis | (21) |
| HCP5 | Human bone marrow mesenchymal stem cells | Functions as miRNA sponge to sequester miR-497, which targets IGF1 to activate IGF1/PI3K/AKT pathway | (22) |
| UCA1 | Human mesenchymal stem cells | Functions as miRNA sponge to sequester miR-873, which targets the anti-apoptotic XIAP | (23) |
| UCA1 | Human umbilical cord mesenchymal stem cells | Functions as miRNA sponge to sequester miR-143, which targets the apoptosis-related gene BCL2 to regulate myocyte survival | (24) |
| circHIPK3 | Hypoxia-induced cardiomyocytes | Functions as miRNA sponge to sequester miR-29a, which targets VEGFA to promote cell cycle progression and proliferation of cardiac endothelial cells | (25) |
exRNAs, extracellular RNAs.
The mammalian adult cardiomyocytes cannot regenerate themselves at a significant rate, if any, to replace the damaged hearts (26). Thus, external ways to regenerate damaged hearts are attractive therapies for CVD. Of such methods, the injection of cells (collectively called as cell therapy) is of particular interest in recent years (27). These cells include mesenchymal cells (multipotent stromal cells) from various cell sources, such as adipocytes, bone marrow, cardiac and skeletal muscle. Originally thought that these injected cells transdifferentiate into cardiomyocytes, it is now widely accepted that the injected cells secrete macromolecules (DNA, RNA, and proteins) that function in a paracrine manner, instead of differentiating into cardiomyocytes (28). Of these paracrine factors, miRNAs, especially those considered as exRNAs, are becoming a hot topic to be investigated (29). For example, exosomes derived from mesenchymal cells isolated from murine bone marrow protected cardiomyocytes in an in vitro oxygen-glucose deprivation model and alleviated the adverse effects in a mouse model of ischemia-reperfusion (I/R) injury (18). Mechanistically, exosomal miR-25-3p targets the pro-apoptotic Fasl and Pten mRNAs to regulate their protein levels as well as the enzymatic component of the polycomb repressive complex 2 (PRC2), Ezh2, in turn derepress the cardioprotective gene eNOS and anti-inflammatory gene Socs3. However, it should be noted that most of these studies use extensively cultured cells (primary cells or cell lines) to produce exRNAs to be injected into the host animals (usually rodents). Furthermore, mesenchymal cells isolated from a tissue source are heterogenous populations of cells. Thus, it is often difficult to pinpoint the observed effects based on one miRNA as there are other factors being involved, including other exRNAs being injected at the same time. Of course, there is no perfect experimental system nor it could also be that the additive effects of several exRNAs, which makes it harder to know the exact mechanism of action of miRNA as exRNA, especially one miRNA has several hundred target mRNAs.
Could lncRNAs be a key to understand exRNAs?
Any non-coding RNAs (ncRNAs) longer than 200 nt are currently categorized as lncRNAs (30-32). Due to this broad definition, the number of lncRNAs is much more than that of protein-coding genes (33). LncRNAs are subcategorized based on their genomic locations to nearby protein-coding genes, such as sense, antisense, intergenic, enhancer, and promoter lncRNAs (34). Their functions are diverse and depend on their macromolecular binding partners (DNA, RNA, and proteins), including recruiting of epigenetic and transcriptional complexes, decoy, splicing, and translational regulations. Furthermore, lncRNAs also found as exRNAs, where it is speculated to contribute to cell-cell communications (35) (Table 2). For example, the lncRNA PVT1 (Pvt1 oncogene) is highly expressed in the exosomes isolated from angiotensin II-induced cultured human cardiac cardiomyocytes (19). Mechanistically, the exosomal PVT1 increased the expression of IL-16 via sequestering miR-145-5p (thus, functioning as miRNA sponge) in macrophages to promote its polarization to pro-inflammatory M1 macrophages.
The nuclear lncRNA, MALAT1 (metastasis associated lung adenocarcinoma transcript 1; also known as NEAT2), is another lncRNA implicated as cardiac exRNA. This abundantly expressed nuclear lncRNA is highly studied in the context of cancer and is conserved among mammals (36). In cardiovascular system, MALAT1 is known to regulate endothelial function and vessel growth (37). Furthermore, Malat1 knockout mice displayed pro-atherosclerotic phenotype due to enhanced accumulation of hematopoietic cells involved in inflammatory responses (38). Not only MALAT1 is functional in nuclei, MALAT1 is secreted as exRNA. MALAT1 in exosomes isolated from endothelial cells is transferred to immature dendritic cells, where MALAT1 interacts with the transcription factor NRF2, thereby inhibits the accumulation of reactive oxygen species and maturation of dendritic cells (20). In a mouse model of atherosclerosis (ApoE knockout mice fed with a high-fat diet for 12 weeks), it was observed that the decreased level of exosomal Malat1 is associated with the progression of atherosclerosis. Another study shows that exosomal MALAT1 isolated from endothelial cells also affects the formation of neutrophil extracellular traps, which is associated with hyperlipidemia and inflammatory response in a mouse model of atherosclerosis (21), further indicating the functional roles of MALAT1 outside of the cellular nuclei.
As it was the case for exosomal miRNAs, lncRNAs are also implicated in cardiac cell therapy as exRNAs. For example, the exosomal lncRNA HCP5 (HLA complex P5) isolated from human bone marrow mesenchymal stem cells increased the viability of cardiomyocytes while decreasing apoptosis in the hearts of rat model of myocardial I/R (22). Mechanistically, HCP5 sequesters miR-497, which targets IGF1 to activate IGF1/PI3K/AKT pathway. Another example is the lncRNA UCA1 (urothelial cancer associated 1). The exosomal UCA1 isolated from human mesenchymal stem cells injected intramyocardially in a rat model of MI showed improved heart functions and reduced fibrosis compared to the PBS-injected rats (23). Mechanistically, UCA1 sponges miR-873, which targets the anti-apoptotic XIAP. The same lncRNA, UCA1, is also reported to bind yet another miRNA when isolated as exosomal lncRNA from human umbilical cord mesenchymal stem cells and injected into a rat model of I/R (24). Similar to the previous study (23), the injection of exosomes showed cardioprotective effects. Mechanistically, UCA1 sponges miR-143, which targets the apoptosis-related gene BCL2 to regulate myocyte survival (24). Among several studies of exosomal lncRNAs in cardiac cell therapy, the lncRNAs functioning as miRNA sponges seem to be a common mechanism. However, as mentioned above, each miRNA targets several hundreds of mRNAs. Furthermore, one lncRNA bind several miRNAs as in the case of UCA1. Thus, it will be necessary to map the signaling pathways being affected by the lncRNA-miRNA-mRNAs axes to understand the effects of mixture of exRNAs being injected to the donor animals.
circRNAs as diagnostic biomarkers
CircRNAs are a subclass of lncRNAs that are generated by backsplicing events of exons and/or introns of both protein-coding and ncRNA genes (39). They lack free ends necessary for exonuclease-mediated degradation. This makes them relatively stable in circulation (40); thus, they are attractive candidates for diagnostic biomarkers, including in CVD (40-46) (Table 2). For example, the circRNA, circHIPK3, is shown to be a key factor in exosomes isolated from hypoxia-induced cardiomyocytes, which regulate oxidative stress damage in cardiac endothelial cells (25). Mechanistically, circHIPK3 sponges miR-29a, which targets VEGFA to promote cell cycle progression and proliferation of cardiac endothelial cells. There are several other studies indicating that exosomal circRNAs function as miRNA sponges in cardiac cells (47-50). However, the current problem in the circRNA research is that the detailed characterization of circRNAs (e.g., genomic locations, biogenesis from the host gene, copy number and the amount in the circulation) is missing in most studies. This is especially problematic when circRNAs are reported as miRNA sponges because circRNAs are expressed at very low level, whereas miRNAs are abundantly present. Thus, it is not clear how one circRNA can sponge the excessive amounts of miRNAs, especially circRNAs are detected as exRNAs in the circulation.
Conclusions
Because of the importance of CVD and the establishment of detection and characterization methods of exRNAs, the number of publications about cardiac exRNAs is rapidly increasing. Although we mainly covered miRNAs, lncRNAs, and circRNAs as cardiac exRNAs here, other exRNAs are increasing being studies, such as tRNA-derived small RNAs (tDRs) with distinct fragmentation signatures in plasma from patients on cardiopulmonary bypass (51) and in children with fulminant myocarditis (52). As mentioned above, even the same miRNA (i.e., miR-155) is indicated to be secreted as exRNAs from the same cell types (i.e., cardiomyocytes and macrophages) to have effects in one another. It is a chicken and egg problem that further research is needed, yet one study used a clear genetic model [i.e., miR-155 knockout mice (17)]. Also, it is not clear how much (in amount or copy number) transfer of exRNAs is needed to have phenotypic effects as suggested because such information is utmost importance to move this field forward beyond using them as potential diagnostic biomarkers of CVD. To solve these problems, more systematic analysis as well as functional and mechanistic studies of cardiac exRNAs are urgently needed to firmly establish the importance of exRNAs as therapeutic tools for CVD.
Acknowledgments
Funding: This work was supported by a grant from the Novo Nordisk Foundation (No. NNF18OC0033438).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chenyu Zhang) for the series “Extracellular RNAs and Human Health” published in ExRNA. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://exrna.amegroups.com/article/view/10.21037/exrna-22-3/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://exrna.amegroups.com/article/view/10.21037/exrna-22-3/coif). The series “Extracellular RNAs and Human Health” was commissioned by the editorial office without any funding or sponsorship. SU serves as an unpaid editorial board member of ExRNA from September 2021 to August 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. Cardiovascular diseases (CVDs) 2021. Available online https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Chiong M, Wang ZV, Pedrozo Z, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis 2011;2:e244. [Crossref] [PubMed]
- Fountoulaki K, Dagres N, Iliodromitis EK. Cellular Communications in the Heart. Card Fail Rev 2015;1:64-8. [Crossref] [PubMed]
- Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 2016;365:563-81. [Crossref] [PubMed]
- Sun K, Li YY, Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct Target Ther 2021;6:79. [Crossref] [PubMed]
- Moore JB, Wysoczynski M. Immunomodulatory Effects of Cell Therapy after Myocardial Infarction. J Cell Immunol 2021;3:85-90. [PubMed]
- Gruner HN, McManus MT. Examining the evidence for extracellular RNA function in mammals. Nat Rev Genet 2021;22:448-58. [Crossref] [PubMed]
- Zernecke A, Preissner KT. Extracellular Ribonucleic Acids (RNA) Enter the Stage in Cardiovascular Disease. Circ Res 2016;118:469-79. [Crossref] [PubMed]
- O'Brien J, Hayder H, Zayed Y, et al. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018;9:402. [Crossref] [PubMed]
- Glinge C, Clauss S, Boddum K, et al. Stability of Circulating Blood-Based MicroRNAs - Pre-Analytic Methodological Considerations. PLoS One 2017;12:e0167969. [Crossref] [PubMed]
- Liu W, Higashikuni Y, Sata M. Linking RNA dynamics to heart disease: the lncRNA/miRNA/mRNA axis in myocardial ischemia-reperfusion injury. Hypertens Res 2022;45:1067-9. [Crossref] [PubMed]
- Wojciechowska A, Braniewska A, Kozar-Kamińska K. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med 2017;26:865-74. [Crossref] [PubMed]
- Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 2015;24:199-206. [Crossref] [PubMed]
- Sadik N, Cruz L, Gurtner A, et al. Extracellular RNAs: A New Awareness of Old Perspectives. Methods Mol Biol 2018;1740:1-15. [Crossref] [PubMed]
- O'Brien K, Breyne K, Ughetto S, et al. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 2020;21:585-606. [Crossref] [PubMed]
- Yu H, Qin L, Peng Y, et al. Exosomes Derived From Hypertrophic Cardiomyocytes Induce Inflammation in Macrophages via miR-155 Mediated MAPK Pathway. Front Immunol 2020;11:606045. [Crossref] [PubMed]
- Wang B, Wang ZM, Ji JL, et al. Macrophage-Derived Exosomal Mir-155 Regulating Cardiomyocyte Pyroptosis and Hypertrophy in Uremic Cardiomyopathy. JACC Basic Transl Sci 2020;5:148-66. [Crossref] [PubMed]
- Peng Y, Zhao JL, Peng ZY, et al. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis 2020;11:317. [Crossref] [PubMed]
- Cao F, Li Z, Ding W, et al. Angiotensin II-Treated Cardiac Myocytes Regulate M1 Macrophage Polarization via Transferring Exosomal PVT1. J Immunol Res 2021;2021:1994328. [Crossref] [PubMed]
- Li H, Zhu X, Hu L, et al. Loss of exosomal MALAT1 from ox-LDL-treated vascular endothelial cells induces maturation of dendritic cells in atherosclerosis development. Cell Cycle 2019;18:2255-67. [Crossref] [PubMed]
- Gao H, Wang X, Lin C, et al. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol Chem 2020;401:367-76. [Crossref] [PubMed]
- Li KS, Bai Y, Li J, et al. LncRNA HCP5 in hBMSC-derived exosomes alleviates myocardial ischemia reperfusion injury by sponging miR-497 to activate IGF1/PI3K/AKT pathway. Int J Cardiol 2021;342:72-81. [Crossref] [PubMed]
- Sun L, Zhu W, Zhao P, et al. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis 2020;11:696. [Crossref] [PubMed]
- Diao L, Zhang Q. Transfer of lncRNA UCA1 by hUCMSCs-derived exosomes protects against hypoxia/reoxygenation injury through impairing miR-143-targeted degradation of Bcl-2. Aging (Albany NY) 2021;13:5967-85. [Crossref] [PubMed]
- Wang Y, Zhao R, Shen C, et al. Exosomal CircHIPK3 Released from Hypoxia-Induced Cardiomyocytes Regulates Cardiac Angiogenesis after Myocardial Infarction. Oxid Med Cell Longev 2020;2020:8418407. [Crossref] [PubMed]
- He L, Nguyen NB, Ardehali R, et al. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress. Circulation 2020;142:275-91. [Crossref] [PubMed]
- Li J, Hu S, Zhu D, et al. All Roads Lead to Rome (the Heart): Cell Retention and Outcomes From Various Delivery Routes of Cell Therapy Products to the Heart. J Am Heart Assoc 2021;10:e020402. [Crossref] [PubMed]
- Sid-Otmane C, Perrault LP, Ly HQ. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J Transl Med 2020;18:336. [Crossref] [PubMed]
- Hunkler HJ, Groß S, Thum T, et al. Non-coding RNAs-key regulators of reprogramming, pluripotency and cardiac cell specification with therapeutic perspective for heart regeneration. Cardiovasc Res 2021; Epub ahead of print. [Crossref] [PubMed]
- Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol 2013;10:925-33. [Crossref] [PubMed]
- Poller W, Dimmeler S, Heymans S, et al. Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J 2018;39:2704-16. [Crossref] [PubMed]
- Beermann J, Piccoli MT, Viereck J, et al. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev 2016;96:1297-325. [Crossref] [PubMed]
- Zhao L, Wang J, Li Y, et al. NONCODEV6: an updated database dedicated to long non-coding RNA annotation in both animals and plants. Nucleic Acids Res 2021;49:D165-71. [Crossref] [PubMed]
- Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res 2015;116:737-50. [Crossref] [PubMed]
- Dragomir M, Chen B, Calin GA. Exosomal lncRNAs as new players in cell-to-cell communication. Transl Cancer Res 2018;7:S243-52. [Crossref] [PubMed]
- Arun G, Aggarwal D, Spector DL. MALAT1 Long Non-Coding RNA: Functional Implications. Noncoding RNA 2020;6:22. [Crossref] [PubMed]
- Michalik KM, You X, Manavski Y, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 2014;114:1389-97. [Crossref] [PubMed]
- Cremer S, Michalik KM, Fischer A, et al. Hematopoietic Deficiency of the Long Noncoding RNA MALAT1 Promotes Atherosclerosis and Plaque Inflammation. Circulation 2019;139:1320-34. Erratum in: Circulation 2019 Jul 16;140(3):e161. [Crossref] [PubMed]
- Kristensen LS, Andersen MS, Stagsted LVW, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 2019;20:675-91. [Crossref] [PubMed]
- Xu S, Zhou L, Ponnusamy M, et al. A comprehensive review of circRNA: from purification and identification to disease marker potential. PeerJ 2018;6:e5503. [Crossref] [PubMed]
- Wang K, Gao XQ, Wang T, et al. The Function and Therapeutic Potential of Circular RNA in Cardiovascular Diseases. Cardiovasc Drugs Ther 2021; Epub ahead of print. [Crossref] [PubMed]
- Liu C, Li N, Dai G, et al. A narrative review of circular RNAs as potential biomarkers and therapeutic targets for cardiovascular diseases. Ann Transl Med 2021;9:578. [Crossref] [PubMed]
- Zhong AN, Yin Y, Tang BJ, et al. CircRNA Microarray Profiling Reveals hsa_circ_0058493 as a Novel Biomarker for Imatinib-Resistant CML. Front Pharmacol 2021;12:728916. [Crossref] [PubMed]
- Wu WP, Pan YH, Cai MY, et al. Plasma-Derived Exosomal Circular RNA hsa_circ_0005540 as a Novel Diagnostic Biomarker for Coronary Artery Disease. Dis Markers 2020;2020:3178642. [Crossref] [PubMed]
- Wang M, Su P, Liu Y, et al. Abnormal expression of circRNA_089763 in the plasma exosomes of patients with post-operative cognitive dysfunction after coronary artery bypass grafting. Mol Med Rep 2019;20:2549-62. [Crossref] [PubMed]
- Kishore R, Garikipati VNS, Gonzalez C. Role of Circular RNAs in Cardiovascular Disease. J Cardiovasc Pharmacol 2020;76:128-37. [Crossref] [PubMed]
- Tian T, Li F, Chen R, et al. Therapeutic Potential of Exosomes Derived From circRNA_0002113 Lacking Mesenchymal Stem Cells in Myocardial Infarction. Front Cell Dev Biol 2021;9:779524. [Crossref] [PubMed]
- Li B, Cai X, Wang Y, et al. Circ-SKA3 Enhances Doxorubicin Toxicity in AC16 Cells Through miR-1303/TLR4 Axis. Int Heart J 2021;62:1112-23. [Crossref] [PubMed]
- Wen Y, Chun Y, Lian ZQ, et al. circRNA-0006896-miR1264-DNMT1 axis plays an important role in carotid plaque destabilization by regulating the behavior of endothelial cells in atherosclerosis. Mol Med Rep 2021;23:311. [Crossref] [PubMed]
- Han J, Zhang L, Hu L, et al. Circular RNA-Expression Profiling Reveals a Potential Role of Hsa_circ_0097435 in Heart Failure via Sponging Multiple MicroRNAs. Front Genet 2020;11:212. [Crossref] [PubMed]
- Li G, Manning AC, Bagi A, et al. Distinct Stress-Dependent Signatures of Cellular and Extracellular tRNA-Derived Small RNAs. Adv Sci (Weinh) 2022;9:e2200829. [Crossref] [PubMed]
- Wang J, Han B, Yi Y, et al. Expression profiles and functional analysis of plasma tRNA-derived small RNAs in children with fulminant myocarditis. Epigenomics 2021;13:1057-75. [Crossref] [PubMed]
Cite this article as: Ilieva M, Uchida S. Extracellular RNAs as communicators in cardiovascular disease: a narrative review. ExRNA 2022;4:14.


