
Extracellular RNAs from immune cells under obesity—a narrative review
Introduction
Extracellular RNAs (exRNAs) perform a variety of cellular functions in many physiological and disease conditions, and our understanding of their role in biology is rapidly advancing. Obesity and its related cardiovascular and metabolic disease components represent a global pandemic affecting hundreds of millions of people worldwide (1-4).
The chronic inflammation and insulin resistance which characterize obesity-related disease are mediated in large part by the immune system and have been extensively studied (5). ExRNAs contribute to inflammation, glucose metabolism, and insulin resistance under obese conditions, and some RNAs are associated with human metabolic disease status (6-8). Additionally, exRNAs derived from immune cells contribute to many of their diverse functions, including antigen presentation, gene expression, T cell priming and costimulation, and monocyte differentiation (9-11). However, the role of immune-derived exRNAs in obesity-related disease is yet to be clearly defined, representing an exciting target for future research (12).
In this review, we describe essential background information on exRNAs produced by the immune system, their role in obesity-related disease, and perspectives on clinical applications and future research directions. We present the following article in accordance with the Narrative Review reporting checklist (available at https://exrna.amegroups.com/article/view/10.21037/exrna-22-15/rc).
Methods
We searched the PubMed database for all scientific publications relevant to the scope of this review on immune-derived exRNAs in obesity-related disease. We filtered for articles published in English prior to May 25, 2022. Our search strategy is outlined in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | May 25, 2022 |
| Databases and other sources searched | PubMed |
| Search terms | ExRNA, obesity, adipose, miRNA, extracellular RNA, exosome, extracellular vesicle, immune |
| Timeframe | Papers published before May 25, 2022 |
| Inclusion and exclusion criteria | We focused on exRNAs derived from immune cells and placed emphasis on publications related to their role in obesity and metabolic disease. Literature in languages other than English was excluded |
ExRNAs from immune cells
Since exRNAs were first reported by Fred Griffith in 1928 (13), they have been the subject of extensive research. Naked exRNAs can be easily degraded by extracellular RNase I, so the most thoroughly studied exRNAs have been those carried by extracellular vesicles (EVs). With the advancement of research, the classification of EVs keeps evolving, but generally, they can be divided into two major categories: exosomes, and ectosomes (microvesicles and macrovesicles) (14).
Accumulated studies demonstrate that exosomes can be produced by most cells, which includes immune cells, such as T cells, B cells, natural killer (NK) cells, monocytes, macrophages, neutrophils, and dendritic cells (DCs). In 2002, Nicolas Blanchard reported that human T cells could produce microvesicles with a diameter of 50–100 nm, bearing TCRβ, CD3ε, and ζ (15). Similar discoveries were made by Roberto Alonso in 2007 of stimulated T cell-secreted exosomes carrying several bio-active proteins (16). Both immature and mature dendritic cells can generate exosomes, through which they can transfer histocompatibility complex (MHC)-peptide complexes to other DCs (9). Activated B cells can secrete exosomes carrying pMHC-II (10).
Composition
Exosomes
Exosomes can be generated by all cell types through inward budding of the endocytic membranes and further forming with intracellular multivesicular bodies (MVB) (17). During this process, intraluminal vesicles (ILVs) are formed, termed “exosomes”. Generally, exosomes, mirroring donor cells, are formed containing any of a multitude of nucleic acids, proteins, and lipids. A shared mechanism of formation and transportation across all the exosomes, which is controlled by endosomal sorting complexes required for transport (ESCRT) proteins such as Alix, TSG101, HSC70, and HSP90β, can be found in all the exosomes (18,19). Tetraspanins and small transmembrane proteins are vital for trapping luminal proteins, which are a common presence in all the exosomes. Other compositions may vary following different cells or variant microenvironments. In one study reported in 1998, enriched CD86, CD37, CD53, CD63, CD81, and CD82 were found in human B cell-derived exosomes through Western blot and electron microscopy (20). In 2005, a study published in Blood reported that exosomes from DCs could express functional MHC class I and II molecules as well as T-cell costimulatory molecules (9). As more advanced methods are adopted in EV research, more exosomal components have been discovered. Different components were compared between healthy and sick patients and treated and untreated groups to explore more therapeutic targets. Kim et al. reported the exosomal miRNAs profiles of patients without diabetes with obesity were altered compared with patients with diabetes with obesity (8). MiR-122, miR-192, miR-27a-3p, and miR-27b-3pre in plasma were confirmed to increase in obese mice (6). Jurkat cells derived exosome was enriched in Valosin-containing protein (VCP), while the exosomes from T cells had higher sphingomyelin and cholesterol, which was reported in a recent lipid-omics analysis (21). Plasma exosomes showed increased oxidative stress markers (cystine, oxidized cys-gly) and decreased polyunsaturated fatty acids (PUFA) in HIV patients (22).
Ectosomes
Ectosomes include microvesicles and macrovesicles. The formation of ectosomes is different from the exosome. The former bud from the plasma membrane, and the latter is from the endocytic membrane. Like exosomes, ectosomes also contain proteins, nucleic acids, and lipids. But they are different from exosomes in specific compositions, even from the same cell, based on the studies carried out by Mathieu et al. (23). The authors identified the presence of LAMP1 was specific to exosomes and that BSG and SLC3A2 were unique to ectosomes through comparative proteomic analysis (23). Ectosomal membranes are enriched in glycoproteins and metalloproteinases. In the new reassessment of the composition of the vesicles, Annexin A1 as a specific marker for microvesicles (ectosome) was identified (19).
Function
Exosomes
Exosomes play important roles in immune activity, such as antigen presentation, activation, immune escape, differentiation, recruitment, and modifying the gene expression of recipient cells. Activated B cells can release exosomes carrying pMHC-II to primed CD4 T cells (10). DCs may adjust their functions through exosome-shuttle miRNAs and MHC-peptide complexes. They can also secrete exosomes to induce antigen-specific T-cell activation. However, the exosome from cancer cells can reduce T-cell activity, cytokine production, and induce apoptosis to achieve immune escape to promote tumor growth (24,25). NK cell-derived exosomes carrying miR-186 can prevent tumor growth and TGFβ1-dependent immune escape in neuroblastoma patients (26). In sum, exosomes, just like a two-edged sword, can strengthen or weaken the human immune system, which will be determined by their donor cells.
Ectosomes
Ectosomes have broad functions from building communication networks, mediating growth, cellular energy transportation, and coagulation. Macrophage-derived microvesicles (ectosomes) can transfer RNA molecules to targeted cells and promote the differentiation of naïve monocytes (11). In recent research, nutrient deprivation-induced ATP-laden microparticles released by melanoma cells can increase extracellular ATP concentration in the colon tumor microenvironment, which can improve the efficacy of anti-cancer therapy (27). It also has been reported that immune cell-derived microparticles can transfer integrin αMβ2 to tumor cells, which can further promote their migration and metastasis (28). In a new trial of cancer treatment, Li et al. found that platelet-derived microparticles can bind to PD-1 receptors to activate T cells in tumor tissue after they combined surgery and tumor-associated macrophages depleting drugs, which may facilitate immunotherapeutic modalities (29). Activated platelets can also secrete microparticles to initiate coagulation (30).
ExRNA action routes
EVs and exRNAs can exert their effects on cells through autocrine, paracrine, and endocrine effects (Figure 1). One study found that breast cancer cells stimulated by fibroblasts enhanced stromal mobilization via an autocrine effect of Wnt11-associated exosomes (31). Another study showed that mouse B16BL6 melanoma cells secreted exosomes to promote growth and inhibit apoptosis (32). Neither of the above studies examined EV RNA contents, though it is likely that exRNA autocrine effects exist and have yet to be described. EV paracrine effects are better established, especially in the context of immune cells, where EVs can assist with antigen presentation and cell activation (33). ExRNA-containing EV endocrine effects are the best studied, likely because of the relative ease of collecting and analyzing blood EVs. For example, EVs containing miR-155 enhanced atherosclerosis progression when secreted by neutrophils (34), and they also increased insulin resistance in obese mice when secreted by adipose tissue macrophages (35). Adipocyte-derived EVs can act as “adipokines” regulating gene expression in distant tissues in mice and humans via a microRNA-mediated mechanism (36). There are many other examples of exRNAs in multiple disease contexts which have been thoroughly reviewed elsewhere (14,37). Circulating, stable miRNAs have also been identified in humans outside of vesicles, though their functions are less clear (38-40).
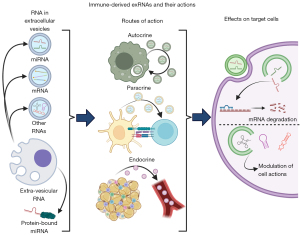
Exosomes interact with target cells through several mechanisms: their primary mechanism of action on target cells is via the effects of delivered cargo; they can interact at the cell surface without delivering cargo, such as in antigen presentation (10,41-43); or they can undergo endocytosis followed by degradation, cargo delivery, or subsequent recycling and secretion (31) (Figure 1). While exosomes can carry a variety of cargoes including protein, lipids, metabolites, DNA, and RNA (44), we will focus on the effects of RNAs in this review.
Nearly every known class of RNA has been found in EVs, including mRNA, miRNA, tRNA, snoRNA, rRNA, and piRNA (45-49). EV RNAs can be selectively determined, as evidenced by the fact EV RNAs differ from overall donor cell RNA composition and that specific RNAs are sorted into EVs (50-53). Extra-vesicular exRNAs are primarily miRNAs, bound to either proteins or lipoproteins such as HDL or LDL cholesterol (38-40).
In both cases, circulating exRNAs must undergo several steps in their interactions with target cells to exert their function (Figure 1). Several excellent, in-depth reviews on the subject are already available (54,55), and thus we will give a basic overview here. First, the RNA must enter the cell, mediated primarily through endocytosis. Other reported mechanisms include direct membrane fusion, micropinocytosis, and phagocytosis (55-58). Uptake of EVs can be non-specific, but it may also be selective (59). Next, endosomal RNA from EVs must leave the endosome and enter the cytosol where it can perform its functions. This represents a major bottleneck in exRNA delivery, as endosomes are primarily targeted for degradation. Additionally, RNAs that escape EVs to either the endosome or the cytosol may activate innate immune receptors, especially as pathogens may use EVs for virulence (60-62).
ExRNAs that enter the cytosol act on target cells largely as they would in the donor cells that produce them. MiRNAs can interact directly with the RNA-induced silencing complex (RISC) from the target cell to perform gene silencing; alternatively, miRNA processing machinery may also be packaged in EVs with miRNAs, though this is controversial (19). EV-derived mRNAs can be translated, though it appears that their contribution to protein expression is negligible (63). tRNAs, one of the most abundant non-coding RNA populations in EVs, can function to modulate immune responses in addition to their roles in protein expression and epigenetics, but their role in target cells remains unclear (47).
Obesity and immune-derived exRNAs
How obesity affects exRNA production from immune cells
Obesity is a risk factor for an expanding set of comorbidities, including cardiovascular disease, type II diabetes mellitus, and non-alcoholic fatty liver disease (5,64,65). White adipose tissue (WAT) dysfunction is central to obesity-associated risk by contributing to systemic, low-grade inflammation and insulin resistance (5,66,67). In lean adipose tissue, resident immune cells maintain tissue fitness; however, WAT inflammation activates and is reinforced by pro-inflammatory immune cell proliferation and infiltration, including macrophages, B cells, T cells, and NK cells (66-69). Adipose tissue-derived exosomes are important to local and systemic metabolic health, particularly through the delivery of microRNAs (6,36,70). Injection of exosomes from obese adipose tissue induced insulin resistance in non-obese mice (6,70) and stimulated pro-inflammatory macrophage polarization (70); however, the cellular origins of these exosomes were not defined. During diet-induced obesity, WAT-exosome content is altered towards a pro-inflammatory profile, particularly for key immune-regulatory microRNAs (6,71), which alter target-cell actions in a lineage-specific manner. Adipose tissue is a significant source of circulating microRNAs in obesity (72). While adipocytes and all immune cells can release and respond to exosomes, adipocytes and macrophages have received the most attention in the context of obesity and exosomal RNA signaling.
Adipose tissue macrophages (ATMs) are the most abundant immune cell within WAT and are crucial to maintaining homeostatic functions through diverse actions (67,73). However, during obesity, pro-inflammatory ATMs are predominant and bolster local inflammation and metabolic dysfunction. Obese ATMs secrete pro-inflammatory mediators including inflammatory cytokines and pro-fibrotic factors that modulate adipocyte insulin sensitivity, extracellular remodeling, adipogenesis, and angiogenesis (66). Emerging evidence supports exosomal RNA transfer between ATMs and adipocytes regulates either’s function (73), suggesting exosomes are an important mode of communication between cells within obese WAT.
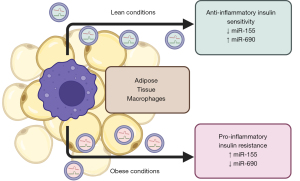
During lean conditions, anti-inflammatory ATMs predominate, and administration of ATM-exosomes from lean WAT ameliorates metabolic dysfunction during diet-induced obesity (35,74). ATM-exosomes’ content from obese and lean conditions are unique in several key microRNAs, including miR-155 and miR-690 (35,74) (Figure 2). Indeed, deletion of the microRNA processor Dicer in macrophages negates the benefit of anti-inflammatory macrophage-exosomes administration under obesity (35). MiR-155 is a ubiquitous immune regulator that modulates macrophage polarization (75) and has been implicated in adipocyte differentiation (76). MiR-155 expression is more abundant in ATM-exosomes during obesity, and injection of these exosomes increased miR-155 levels in metabolic tissues including adipocytes, muscle, and liver (35). In opposition Ying et al. (74) showed that miR-690 is enriched in exosomes from anti-inflammatory ATMs and in lean conditions and is likewise increased in metabolic tissues following injection of miR-690-containing exosomes. Exosomal delivery of miR-690 alone ameliorated metabolic dysfunction, in part, through repressing NADH kinase (Nadk) in adipocytes (74).
Adipocyte-exosomal microRNAs regulate local and systemic inflammation and metabolism in part by modulating immune cell recruitment and activation. Adipocyte-specific deletion of the microRNA processor Dicer significantly impaired adipose tissue functions and altered plasma exosomal content (36). MiR-27a is increased in serum of obese humans and animal models and elevated specifically in adipocyte-exosomes under obesity (6,77). MiR-27a can repress translation of Peroxisome proliferator-activated receptor gamma (PPAR-γ), an important transcription factor in adipogenesis, mature adipocyte lipid metabolism, and macrophage polarization (77-80). Although not studied in the context of exosomes, miR-27a expression modulates adipocyte glucose utilization via PPAR-γ repression (81), and knock-down ameliorates metabolic dysfunction during obesity (82). In addition, adipocytes cultured in excessive lipid levels secreted abundant miR-27a and instigated macrophage migration, an effect abrogated by miR-27a inhibitors (82). Likewise, Pan et al. (83) found that miR-34a is highly expressed in mature adipocytes and is elevated in obese humans and mouse models. In this study, adipocyte-specific deletion of miR-34a improved metabolic function under diet-induced obesity in a macrophage-dependent manner. The authors found that adipocyte-exosomes from obese mice were enriched in miR-34a, which were confirmed to be taken up by macrophages and induced a subsequent increase in miR-34a expression. Extended incubation with adipocyte-derived exosomes from obese conditions impeded anti-inflammatory macrophage polarization, an effect mediated by miR-34a repression of anti-inflammatory transcription factor Kruppel-like factor 4 (Klf4) (83).
Although less studied, NK cells also contribute to WAT inflammation during obesity through pro-inflammatory mediators. Exosomal delivery of miR-1249-3p from NK cells to adipocytes has been implicated in supporting metabolic health by repressing SKI family transcriptional corepressor 1 (SKOR1), a regulator of pro-inflammatory signaling (84). These studies urge further investigations into exosomal-RNAs and microRNA exchange between adipose tissue resident immune cells and adipocytes in obesity, presenting a targetable mechanisms of obesity-induced WAT dysfunction.
exRNAs in obesity-related disease
Epidemiological studies have investigated the association of exosomal content across healthy individuals and those with obesity-associated conditions (7); however, whether observed changes contribute to disease or are a consequence must be established. Obesity increases the risk of non-alcoholic fatty liver disease (NAFLD), a spectrum of conditions ranging from triglyceride accumulation in hepatocytes (steatosis) to liver fibrosis that significantly impairs liver function. Infiltration of pro-inflammatory macrophages plays a central role in the development of inflammation that leads to NAFLD and liver fibrosis (5,85). Serum exosomes are more abundant in NAFLD patients and correlated with disease severity. Both serum exosomes and the exosomes from hepatocytes cultured under NAFLD-like conditions are abundant in miR-192-5p (86). Macrophages cultured with miR-192-5p-containing hepatocyte-exosomes became pro-inflammatory through targeting of Rapamycin-insensitive companion of mTOR (Rictor), demonstrating an exosome-dependent mechanism that accelerates NAFLD progression (86). Further, exosomes from IL-6 stimulated macrophages can impede NAFLD via delivery of miR-223, a repressor of pro-fibrotic factor Tafazzin (TAZ) (87).
Cardiovascular disease causes significant mortality each year and is a co-morbidity of obesity (2-4). While the role of exosomes from circulating immune cells, endothelial cells, and vascular smooth muscle cells has been investigated (88), the link between obesity-related changes in exosome abundance and content has not been well established. Further, in the case of Type II diabetes, while several studies have sought to determine exosomal content as biomarkers for obesity-induced pathogenesis, the majority are done in patients suffering from both obesity and diabetes, complicating interpretations (89). Thus, more nuanced investigations into lineage-specific exosomes altered in obesity and their systemic target cells are needed to elucidate their impact on these obesity-induced diseases.
Clinical applications of exRNAs
The clinical applications of exRNAs are broad and actively being researched. Perhaps the most famous example of clinical exRNA usage is in the recent development of mRNA-based SARS-CoV-2 vaccines delivered by lipid nanoparticles (90). However, in any mRNA delivery method, including EVs, activation of innate immune receptors is a major obstacle to overcome (46,60). Extracellular miRNAs have most commonly been investigated as biomarkers, and they are excellent targets for biomarker research because they are relatively protected from degradation by either the EVs that contain them or their protein carriers. Serum miR-223, for example, has been suggested for use as a biomarker for obesity (91), though there are many other relevant diseases. For further reading on EVs in human disease, we recommend the following review (37).
Extracellular miRNAs also hold significant therapeutic potential. EVs carrying a variety of molecules have been investigated for their use in cancer, immune-modulatory, regenerative, and even infectious disease therapies (92). Because EVs are non-immunogenic, enter cells relatively easily, are well-tolerated, and protect cargo from degradation, they are popular as vehicles for drug delivery (46,92). EVs specifically loaded with RNA also have promising clinical applications. Many studies have applied naturally-derived miRNA-containing EVs therapeutically, and miRNA-mimicking siRNAs and shRNAs have also been used to inhibit gene expression in target cells (93,94). For further reading on therapeutic applications and the associated technical challenges, we recommend the following reviews (46,95,96). In spite of the broad and well-characterized clinical applications of exRNAs and their known role in regulating insulin and glucose tolerance, their use for obesity and metabolic disease treatment has thus far been limited (6,14,35,55). Therefore, we believe that exRNAs could serve as a critical avenue for further clinical investigation.
Concluding remarks
ExRNAs perform critical functions to modulate cell actions under both healthy and disease conditions. Due to rapid technical advances, our understanding of exRNA and EV biology have increased tremendously in recent years, and we anticipate further improvements in the ability to characterize exRNAs and the EVs that may carry them in the near future. While there are several high-quality experimental studies demonstrating the profound effects of exRNAs derived from immune cells in models of obesity-related disease, these studies are relatively few in number. We hope that they will serve as a foundation for further work to investigate the mechanisms through which exRNAs impact metabolic disease. We also hope for more effective synthesis of exRNAs and EVs for therapeutic use, representing a promising avenue to limit the global impact from obesity and its related diseases.
Acknowledgments
Figures 1 and 2 were created with BioRender.com.
Funding: Funding support was provided by American Heart Association (grant No. 19TPA34910079) and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (No. R01DK121805) to Beiyan Zhou.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chenyu Zhang) for the series “Extracellular RNAs and Human Health” published in ExRNA. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://exrna.amegroups.com/article/view/10.21037/exrna-22-15/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://exrna.amegroups.com/article/view/10.21037/exrna-22-15/coif). The series “Extracellular RNAs and Human Health” was commissioned by the editorial office without any funding or sponsorship. BZ reports funding support from American Heart Association (grant No. 19TPA34910079) and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (No. R01DK121805). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018;17:83. [Crossref] [PubMed]
- Singh GM, Danaei G, Farzadfar F, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One 2013;8:e65174. [Crossref] [PubMed]
- Hubert HB, Feinleib M, McNamara PM, et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67:968-77. [Crossref] [PubMed]
- Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol 2019;7:231-40. [Crossref] [PubMed]
- Frühbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol 2008;456:1-22. [Crossref] [PubMed]
- Castaño C, Kalko S, Novials A, et al. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A 2018;115:12158-63. [Crossref] [PubMed]
- Karolina DS, Tavintharan S, Armugam A, et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab 2012;97:E2271-6. [Crossref] [PubMed]
- Kim H, Bae YU, Lee H, et al. Effect of diabetes on exosomal miRNA profile in patients with obesity. BMJ Open Diabetes Res Care 2020;8:e001403. [Crossref] [PubMed]
- Segura E, Nicco C, Lombard B, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005;106:216-23. [Crossref] [PubMed]
- Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J 2007;26:4263-72. [Crossref] [PubMed]
- Ismail N, Wang Y, Dakhlallah D, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013;121:984-95. [Crossref] [PubMed]
- Li C, Qu L, Farragher C, et al. MicroRNA Regulated Macrophage Activation in Obesity. J Transl Int Med 2019;7:46-52. [Crossref] [PubMed]
- Griffith F. The Significance of Pneumococcal Types. J Hyg (Lond) 1928;27:113-59. [Crossref] [PubMed]
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367:eaau6977. [Crossref] [PubMed]
- Blanchard N, Lankar D, Faure F, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol 2002;168:3235-41. [Crossref] [PubMed]
- Alonso R, Mazzeo C, Mérida I, et al. A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie 2007;89:213-21. [Crossref] [PubMed]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255-89. [Crossref] [PubMed]
- van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19:213-28. [Crossref] [PubMed]
- Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell 2019;177:428-445.e18. [Crossref] [PubMed]
- Escola JM, Kleijmeer MJ, Stoorvogel W, et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 1998;273:20121-7. [Crossref] [PubMed]
- Bosque A, Dietz L, Gallego-Lleyda A, et al. Comparative proteomics of exosomes secreted by tumoral Jurkat T cells and normal human T cell blasts unravels a potential tumorigenic role for valosin-containing protein. Oncotarget 2016;7:29287-305. [Crossref] [PubMed]
- Chettimada S, Lorenz DR, Misra V, et al. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci Rep 2018;8:7227. [Crossref] [PubMed]
- Mathieu M, Névo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun 2021;12:4389. [Crossref] [PubMed]
- Kim DH, Kim H, Choi YJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med 2019;51:1-13. [Crossref] [PubMed]
- Vignard V, Labbé M, Marec N, et al. MicroRNAs in Tumor Exosomes Drive Immune Escape in Melanoma. Cancer Immunol Res 2020;8:255-67. [Crossref] [PubMed]
- Neviani P, Wise PM, Murtadha M, et al. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res 2019;79:1151-64. [Crossref] [PubMed]
- Vultaggio-Poma V, Falzoni S, Chiozzi P, et al. Extracellular ATP is increased by release of ATP-loaded microparticles triggered by nutrient deprivation. Theranostics 2022;12:859-74. [Crossref] [PubMed]
- Ma J, Cai W, Zhang Y, et al. Innate immune cell-derived microparticles facilitate hepatocarcinoma metastasis by transferring integrin α(M)β2 to tumor cells. J Immunol 2013;191:3453-61. [Crossref] [PubMed]
- Li Z, Ding Y, Liu J, et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun 2022;13:1845. [Crossref] [PubMed]
- Keuren JF, Magdeleyns EJ, Govers-Riemslag JW, et al. Effects of storage-induced platelet microparticles on the initiation and propagation phase of blood coagulation. Br J Haematol 2006;134:307-13. [Crossref] [PubMed]
- Luga V, Zhang L, Viloria-Petit AM, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 2012;151:1542-56. [Crossref] [PubMed]
- Matsumoto A, Takahashi Y, Nishikawa M, et al. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer Sci 2017;108:1803-10. [Crossref] [PubMed]
- Montecalvo A, Larregina AT, Shufesky WJ, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012;119:756-66. [Crossref] [PubMed]
- Gomez I, Ward B, Souilhol C, et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat Commun 2020;11:214. [Crossref] [PubMed]
- Ying W, Riopel M, Bandyopadhyay G, et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017;171:372-384.e12. [Crossref] [PubMed]
- Thomou T, Mori MA, Dreyfuss JM, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017;542:450-5. [Crossref] [PubMed]
- Shah R, Patel T, Freedman JE. Circulating Extracellular Vesicles in Human Disease. N Engl J Med 2018;379:958-66. [Crossref] [PubMed]
- Wang K, Zhang S, Weber J, et al. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 2010;38:7248-59. [Crossref] [PubMed]
- Mori MA, Ludwig RG, Garcia-Martin R, et al. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab 2019;30:656-73. [Crossref] [PubMed]
- Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 2011;13:423-33. [Crossref] [PubMed]
- Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183:1161-72. [Crossref] [PubMed]
- Denzer K, van Eijk M, Kleijmeer MJ, et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol 2000;165:1259-65. [Crossref] [PubMed]
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011;2:282. [Crossref] [PubMed]
- Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847-56. [Crossref] [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- O'Brien K, Breyne K, Ughetto S, et al. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 2020;21:585-606. [Crossref] [PubMed]
- Chiou NT, Kageyama R, Ansel KM. Selective Export into Extracellular Vesicles and Function of tRNA Fragments during T Cell Activation. Cell Rep 2018;25:3356-3370.e4. [Crossref] [PubMed]
- Xiang Y, Zhang J, Huang K. Mining the tissue-tissue gene co-expression network for tumor microenvironment study and biomarker prediction. BMC Genomics 2013;14:S4. [Crossref] [PubMed]
- Chakrabortty SK, Prakash A, Nechooshtan G, et al. Extracellular vesicle-mediated transfer of processed and functional RNY5 RNA. RNA 2015;21:1966-79. [Crossref] [PubMed]
- Nolte-'t Hoen EN, Buermans HP, Waasdorp M, et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res 2012;40:9272-85. [Crossref] [PubMed]
- Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genomics 2011;12:S18. [Crossref] [PubMed]
- Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 2013;4:2980. [Crossref] [PubMed]
- Wei H, Chen Q, Lin L, et al. Regulation of exosome production and cargo sorting. Int J Biol Sci 2021;17:163-77. [Crossref] [PubMed]
- Bartel DP. Metazoan MicroRNAs. Cell 2018;173:20-51. [Crossref] [PubMed]
- Isaac R, Reis FCG, Ying W, et al. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab 2021;33:1744-62. [Crossref] [PubMed]
- Horibe S, Tanahashi T, Kawauchi S, et al. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 2018;18:47. [Crossref] [PubMed]
- Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 2014; [Crossref] [PubMed]
- Tian T, Zhu YL, Zhou YY, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 2014;289:22258-67. [Crossref] [PubMed]
- Romani P, Brian I, Santinon G, et al. Extracellular matrix mechanical cues regulate lipid metabolism through Lipin-1 and SREBP. Nat Cell Biol 2019;21:338-47. [Crossref] [PubMed]
- Boulding T, McCuaig RD, Tan A, et al. LSD1 activation promotes inducible EMT programs and modulates the tumour microenvironment in breast cancer. Sci Rep 2018;8:73. [Crossref] [PubMed]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 2010;107:6328-33. [Crossref] [PubMed]
- Feng Z, Hensley L, McKnight KL, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 2013;496:367-71. [Crossref] [PubMed]
- Hung ME, Leonard JN. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J Extracell Vesicles 2016;5:31027. [Crossref] [PubMed]
- GBD 2015 Obesity Collaborators; Afshin A, Forouzanfar MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017;377:13-27.
- Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161:1581-6. [Crossref] [PubMed]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175-84. [Crossref] [PubMed]
- Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796-808. [Crossref] [PubMed]
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A 2013;110:5133-8. [Crossref] [PubMed]
- Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 2011;17:610-7. [Crossref] [PubMed]
- Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009;58:2498-505. [Crossref] [PubMed]
- Wei M, Gao X, Liu L, et al. Visceral Adipose Tissue Derived Exosomes Exacerbate Colitis Severity via Pro-inflammatory MiRNAs in High Fat Diet Fed Mice. ACS Nano 2020;14:5099-110. [Crossref] [PubMed]
- Hevener AL, He W, Barak Y, et al. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 2003;9:1491-7. [Crossref] [PubMed]
- Matz A, Qu L, Karlinsey K, et al. Impact of microRNA Regulated Macrophage Actions on Adipose Tissue Function in Obesity. Cells 2022;11:1336. [Crossref] [PubMed]
- Ying W, Gao H, Dos Reis FCG, et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab 2021;33:781-790.e5. [Crossref] [PubMed]
- Arranz A, Doxaki C, Vergadi E, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A 2012;109:9517-22. [Crossref] [PubMed]
- Chen Y, Siegel F, Kipschull S, et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 2013;4:1769. [Crossref] [PubMed]
- Yu Y, Du H, Wei S, et al. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics 2018;8:2171-88. [Crossref] [PubMed]
- Lin Q, Gao Z, Alarcon RM, et al. A role of miR-27 in the regulation of adipogenesis. FEBS J 2009;276:2348-58. [Crossref] [PubMed]
- Galhardo M, Sinkkonen L, Berninger P, et al. Integrated analysis of transcript-level regulation of metabolism reveals disease-relevant nodes of the human metabolic network. Nucleic Acids Res 2014;42:1474-96. [Crossref] [PubMed]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell 2005;123:993-9. [Crossref] [PubMed]
- Chen T, Zhang Y, Liu Y, et al. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging (Albany NY) 2019;11:7510-24. [Crossref] [PubMed]
- Yao F, Yu Y, Feng L, et al. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARγ of insulin resistance induced by high-fat diet-associated obesity. Exp Cell Res 2017;355:105-12. [Crossref] [PubMed]
- Pan Y, Hui X, Hoo RLC, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest 2019;129:834-49. [Crossref] [PubMed]
- Wang Y, Li M, Chen L, et al. Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes. Signal Transduct Target Ther 2021;6:409. [Crossref] [PubMed]
- Kazankov K, Jørgensen SMD, Thomsen KL, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2019;16:145-59. [Crossref] [PubMed]
- Liu XL, Pan Q, Cao HX, et al. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology 2020;72:454-69. [Crossref] [PubMed]
- Hou X, Yin S, Ren R, et al. Myeloid-Cell-Specific IL-6 Signaling Promotes MicroRNA-223-Enriched Exosome Production to Attenuate NAFLD-Associated Fibrosis. Hepatology 2021;74:116-32. [Crossref] [PubMed]
- Wang C, Li Z, Liu Y, et al. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics 2021;11:3996-4010. [Crossref] [PubMed]
- Pardo F, Villalobos-Labra R, Sobrevia B, et al. Extracellular vesicles in obesity and diabetes mellitus. Mol Aspects Med 2018;60:81-91. [Crossref] [PubMed]
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020;586:594-9. [Crossref] [PubMed]
- Wen D, Qiao P, Wang L. Circulating microRNA-223 as a potential biomarker for obesity. Obes Res Clin Pract 2015;9:398-404. [Crossref] [PubMed]
- Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015;4:30087. [Crossref] [PubMed]
- El Andaloussi S, Lakhal S, Mäger I, et al. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev 2013;65:391-7. [Crossref] [PubMed]
- Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017;546:498-503. [Crossref] [PubMed]
- Wiklander OPB, Brennan MÁ, Lötvall J, et al. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med 2019;11:eaav8521. [Crossref] [PubMed]
- Winkle M, El-Daly SM, Fabbri M, et al. Noncoding RNA therapeutics - challenges and potential solutions. Nat Rev Drug Discov 2021;20:629-51. [Crossref] [PubMed]
Cite this article as: Karlinsey K, Matz A, Qu L, Zhou B. Extracellular RNAs from immune cells under obesity—a narrative review. ExRNA 2022;4:18.


